Selected publications
Human effector CD8+ T cells with an activated and exhausted-like phenotype control tumour growth in vivo in a humanized tumour model
Mietz J, Kaulfuss M, Egli L, Opitz L, Münz C, Chijioke O
eBioMedicine (2024)
DOI: 10.1016/j.ebiom.2024.105240
Abstract
We established an autologous humanized mouse tumour model by using NSG mice reconstituted with human immune cells from hematopoietic progenitors and tumours generated from transformed autologous human B cells. We demonstrate growth of solid lymphoid tumours after subcutaneous implantation, infiltration by endogenous human immune cells and immunocompetence of the model. The autologous humanized tumour model provided in this study can serve as a tool to elucidate human-specific immune features. By bridging a gap between syngeneic mouse tumour models and human-specific antitumour immune responses, the model may help open up avenues for greater translatability of preclinical data. Our findings suggest that exhausted-like effector CD8+ T cells can be harnessed for clinical development of adoptive T cell therapies.
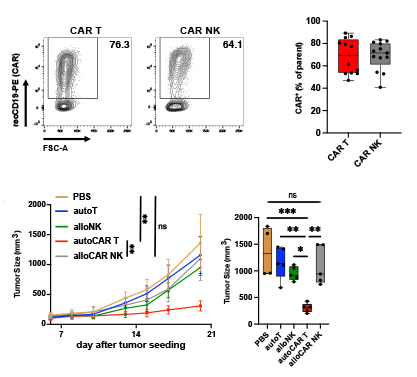
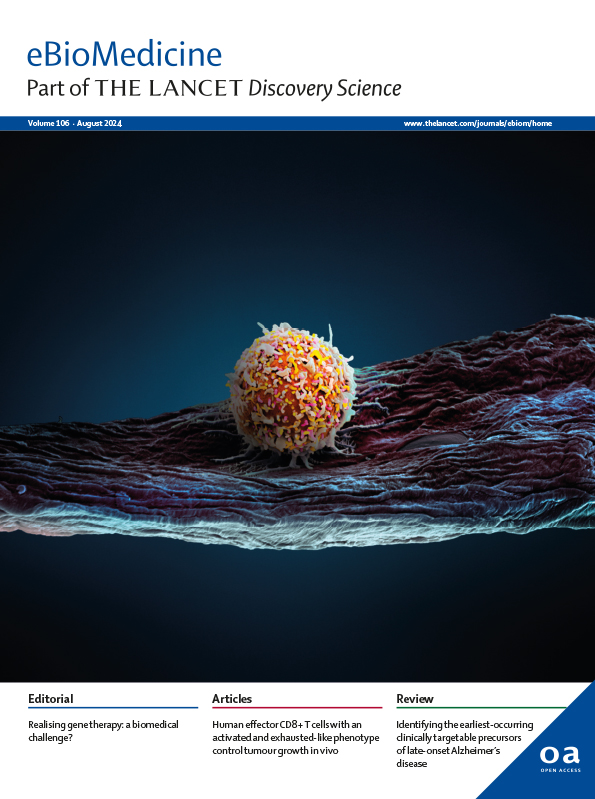
CAR T cells outperform CAR NK cells in CAR-mediated effector functions in head-to-head comparison
Egli L, Kaulfuss M, Mietz J, Picozzi A, Verhoeyen E, Münz C, Chijioke O
Experimental Hematology & Oncology (2024)
DOI: 10.1186/s40164-024-00522-6
Abstract
CAR NK cells as vehicles for engineered “off-the-shelf” cellular cancer immunotherapy have attracted significant interest. Nonetheless, a comprehensive comparative assessment of the anticancer activity of CAR T cells and CAR NK cells carrying approved benchmark anti-CD19 CAR constructs is missing. Here, we report a direct head-to-head comparison of CD19-directed human T and NK cells. Our main findings are a drastically reduced capacity for CAR-mediated IFN-γ production and lower CAR-mediated cytotoxicity of CAR NK cells relative to CAR T cells in vitro. Consistent with these in vitro findings, we report superior anticancer activity of autologous CAR T cells compared with allogeneic CAR NK cells in vivo.
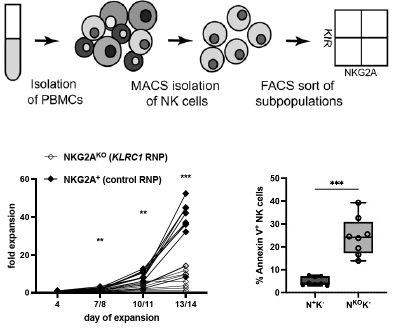
The NK cell checkpoint NKG2A maintains expansion capacity of human NK cells
Kaulfuss M, Mietz J, Fabri A, Vom Berg J, Münz C, Chijioke O
Scientific Reports (2023)
DOI: 10.1038/s41598-023-37779-6
Abstract
Human natural killer (NK) cells are cytotoxic effector cells that are increasingly harnessed in cancer immunotherapy. NKG2A/CD94 is an inhibitory receptor on NK cells that has established regulatory functions in the direct interaction with target cells when engaged with its ligand, the non-classical HLA class I molecule HLA-E. Here, we confirmed NKG2A as a checkpoint molecule in primary human NK cells and identified a novel role for NKG2A in maintaining NK cell expansion capacity by dampening both proliferative activity and excessive activation-induced cell death. Maintenance of NK cell expansion capacity might contribute to the preferential accumulation of human NKG2A+ NK cells after hematopoietic cell transplantation and enrichment of functionally impaired NK cells in human cancers. Functional silencing of NKG2A for cancer immunotherapy is highly attractive but will need to consider that this might also lead to a reduced survival by driving activation-induced cell death in targeted NK cells.